南湖新闻网讯(通讯员 刘潇)近日,我校植物科学技术学院农药毒理团队研究成果以Adsorption behavior of azole fungicides on polystyrene and polyethylene microplastics为题在Chemosphere上在线发表。该研究定量解析了农用塑料薄膜降解颗粒对三唑类杀菌剂的吸附过程,探明了影响吸附过程的关键环境因子,基于一系列表征分析与多种热力学/动力学吸附模型,揭示了塑料颗粒对供试农药的吸附机理。
农用塑料薄膜广泛应用于农业生产过程,起着提高地温、保持土壤湿度、抑制杂草生长等作用。研究表明,塑料农膜在田间暴露下会分解成微米级塑料颗粒(MPs)。这些微米塑料具有可观的比表面积和丰富的官能团,使其拥有强力的吸附性能,它们暴露在农田系统中可能会吸附环境中的农药分子,改变其生物活性,影响实际应用效果,并使得农药残留的污染更容易在生态系统中持久和传播。
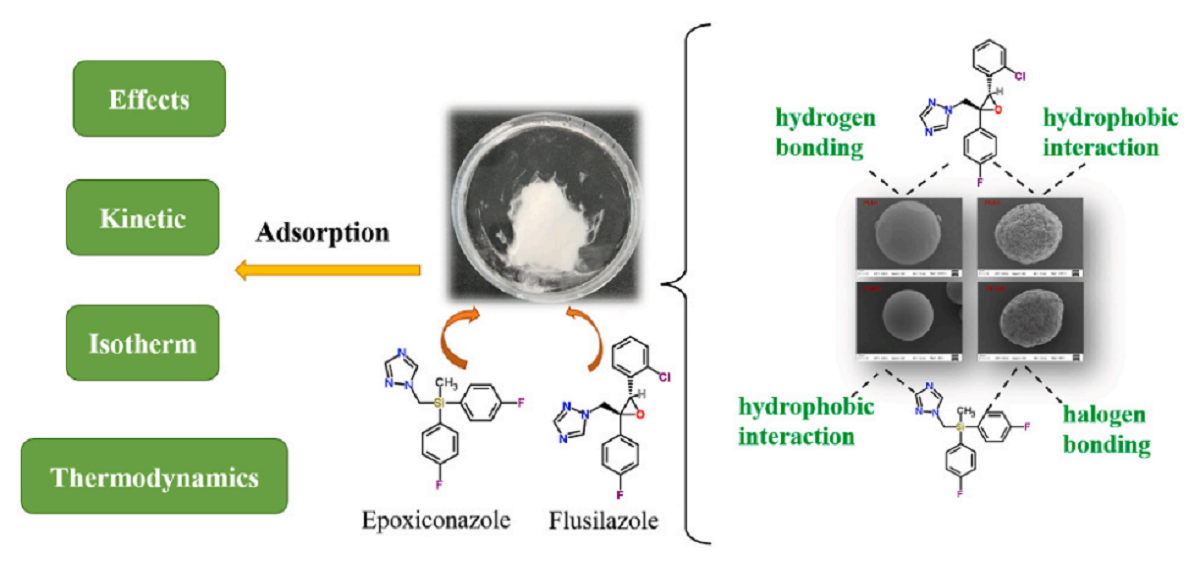
三唑类杀菌剂在聚苯乙烯和聚乙烯微塑料上的吸附行为
研究团队以环境中检出率较高的两种微塑料(聚乙烯和聚苯乙烯)及覆膜栽培作物玉米上常用的两种杀菌剂(氟硅唑和氟环唑)为研究对象,定量解析微塑料对目标农药的吸附行为及机理。研究表明,微塑料的理化性质(结晶度、粒径)和农药的性质(疏水性)以及环境因素(pH、离子强度、溶解性有机质、混合体系等)都会影响微塑料对农药的吸附。通过吸附动力学模型、吸附等温线模型、吸附热力学模型以及一系列表征分析技术,证实了吸附过程中可能发生表面吸附、传质和颗粒内扩散,属于不均匀表面上的多层吸附过程。此外,氢键、卤素键和疏水相互作用在吸附中具有重要作用。农药施用后往往不是单一暴露于环境中,而是与其他化合物共同暴露。本研究将农田系统中普遍残留的塑料颗粒与农药结合,为科学开展农药生物活性评价、合理使用塑料农膜提供理论依据。
植物科学技术学院资源利用与植物保护专业硕士研究生刘潇为该论文第一作者,杨中华副教授为通讯作者,硕士研究生周冬冬、陈敏、曹议文、庄绿韵等也参加了部分工作。该研究得到国家重点研发计划、中国农业产业研究专项基金等项目资助。
审核人:李建洪
【英文摘要】
Agricultural plastic films and triazole fungicides are widely used in agricultural production process. Exposure to natural environment, agricultural plastic films will degrade into micron plastic particles, which will adsorb pesticide molecules and may affect their toxicity, biological activity and persistence. The long-term coexistence of microplastics (MPs) and triazole fungicides will bring potential harms to the agricultural ecological environment. Therefore, two kinds of triazole fungicides flusilazole (FLU) and epoxiconazole (EPO) were selected as cases and the adsorption behaviors of them on polystyrene and polyethylene were investigated. A series of factors which could affect the adsorption behavior were evaluated. Specifically, the particle size of MPs could affect its adsorption capacity, and the smaller the particle size, the stronger the adsorption capacity. Moreover, with the increase of pH value from 6.0 to 9.0, the adsorption capacity of MPs to target compounds gradually increased. The effect of ionic strength was evaluated by NaCl, and 0.05% of NaCl was beneficial to the adsorption process, while the continuous increase of NaCl concentration inhibited the adsorption. Oxalic acid and humic acid decreased the adsorption capacity of flusilazole on PE by 15.99–32.00% and PS by 35.02–48.67%, respectively. In addition, compared with the single pesticide system, the adsorption capacity of MPs for flusilazole and epoxiconazole in the binary pesticides system decreased by 36.13–37.93% and 44.36–51.35%, respectively, indicating that competitive adsorption occurred between the two pesticides. Meanwhile, the adsorption process was evaluated by adsorption kinetics and adsorption isotherms and were consistent with pseudo-second-order kinetic model and Freundlich isotherm model, respectively. Finally, several characterization analyses were conducted to investigated the adsorption mechanism, and hydrogen, halogen bonding and hydrophobic interaction proved to play an important role. The study on the adsorption behavior and mechanism of pesticide on MPs was the basis of assessing the risk of joint exposure.
论文链接:https://www.sciencedirect.com/science/article/pii/S0045653522027734
