南湖新闻网讯(通讯员 张怡)近日,我校生物地质矿化研究课题组针对纳米尺度粘土界面上磷素的界面行为和物质循环这一课题开展系统深入的研究,相关成果分别以“Adsorption effects and mechanisms of phosphorus by nanosized laponite”和“Molecular mechanisms of phosphorus immobilization by nano-clay mediated by dissolved organic matter”为题发表在Chemosphere和Chemical Geology上。
磷是土壤体系中广泛存在的植物必需营养元素之一,在生物系统中具有诸多不可或缺的作用。目前研究认为复杂的土壤成分以及多变的土壤环境因素对磷的生物地球化学循环过程具有重要的调控作用,同时农业生产活动以及生产中废水的排放也严重影响了环境中磷的循环过程。因此,探究磷在环境界面的行为和物质循环对于提高磷肥的有效性、控制面源污染以及潜在的磷资源回收与再利用具有重要的意义。
粘土是土壤中含量占比最大的组分,被认为是天然的磷贮藏库,纳米尺度粘土相较于大粒径的粘土具有更大的比表面积,可能具有更强的磷固定能力。受限于研究手段,目前就纳米粘土调控磷循环过程的研究尚不清楚。因此,生物-地质矿化研究课题组选择尺寸均一、结构清晰的纳米硅酸镁锂作为代表性纳米粘土,将吸附试验和原子力显微镜、透射电镜、Zeta电位分析等表征手段相结合,探究了环境中不同无机、有机因子介导下纳米粘土对有机/无机磷吸附能力的机制。
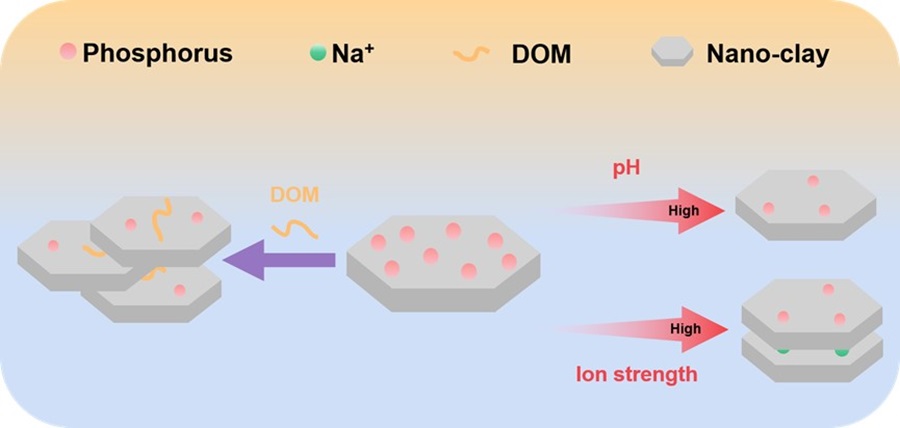
不同环境因子调控纳米粘土磷吸附的示意图
结果表明,在不同pH、离子强度条件的影响下,纳米粘土表面和层间可通过氢键吸附无机磷酸盐和有机植酸,随着pH增加,纳米粘土与磷素的氢离子解离导致其负电荷增加进而增大分子间静电斥力,抑制磷吸附作用,且离子强度通过减弱纳米粘土与磷素之间的静电斥力和纳米粘土的粒径调控纳米粘土对磷素的吸附。以腐殖酸为代表的土壤有机质通过竞争粘土矿物上的吸附位点及促进纳米粘土团聚,抑制纳米粘土对磷的吸附固定效果,这说明土壤中有机质的存在可以促进土壤磷素的有效性。在此基础上,通过动力学力谱解析了上述环境因子作用下磷素和纳米粘土的结合强度,并从单分子尺度剖析了环境因子作用下磷与纳米粘土作用的热力学机制。研究成果丰富了土壤中磷素生物地球化学循环的研究体系,为促进磷的可持续利用性提供了新的理论依据。
我校资源与环境学院硕士研究生贾崇昊为论文第一作者,张文君副教授为通讯作者,广东省科学院生态环境与土壤研究所迟家霖博士参与了本研究的工作,为共同通讯作者,上述研究得到国家自然科学基金、国家重点研发计划的支持。
审核人 张文君
【英文摘要】
Chemosphere:
Phosphorus (P), an important macroelement for crops, may be lost into water systems by human activities and subsequently cause serious environmental problems such as eutrophication. Thus, the recovery of P from wastewater is essential. P can be adsorbed and recovered from wastewater using many natural, environmentally friendly clay minerals, however the adsorption ability is limited. Here we applied a synthesis nanosized clay mineral, laponite, to evaluate the P adsorption ability and molecular mechanisms of the adsorption process. We apply X-ray Photoelectron Spectroscopy (XPS) to observe the adsorption of inorganic phosphate onto laponite, and then measure the adsorption content of phosphate by laponite via batch experiments in different solution conditions, including pH, ionic species and concentrations. Then the molecular mechanisms of adsorption are analyzed by Transmission Electron Microscopy (TEM) and molecular modeling using Density Functional Theory (DFT). The results show that phosphate adsorbs to the surface and interlayer of laponite via hydrogen bonding, and the adsorption energies of the interlayer are greater than those of the surface. These bulk solution and molecular-scale results in a model system may provide new insights into the recovery of phosphorus by nanosized clay, with possible environmental engineering applications for P-pollution control and sustainable utilization of P sources.
Chemical Geology:
The adsorption of phosphorus (P) by minerals, particularly clay minerals, plays a crucial role in the biogeochemical cycling of P in geological environments such as soil and groundwater. However, the mechanisms underlying the immobilization and subsequent release of element P by nano-clay are still not well comprehended. This study delves into the mechanism of adsorption of inorganic phosphate and organic phytate onto laponite, serving as a representative nano-clay. This aspect has frequently been disregarded due to research method limitations; nevertheless, the substantial specific surface areas (SSA) are abundant in a multitude of potential adsorption sites. Batch experiments were conducted to evaluate surface reaction and adsorption capacity under varying solution conditions, including pH, ionic strength, and the presence of dissolved organic matter (DOM). The results demonstrate that the adsorption capacity of laponite decreases with increasing pH. Additionally, the adsorption capacity initially increases within the range of 10-50 mM NaCl and then decreases within 50-100 mM NaCl, while the presence of DOM significantly inhibits P adsorption. Complementary techniques such as X-ray photoelectron spectroscopy (XPS), zeta potential analysis, atomic force microscopy (AFM) were employed to gain further insights into the adsorption process. These analyses revealed that DOM obstructs P adsorption by occupying adsorption sites and promoting the aggregation of laponite nanoparticles. Furthermore, AFM-based dynamic force spectroscopy (DFS) provides molecular-scale thermodynamic support for the observed P adsorption phenomena on laponite under different conditions. The findings of this study contribute to a better understanding of the P adsorption mechanism of nano-clay and have valuable implications for the biogeochemical cycling of P in soil and groundwater.