
南湖新闻网讯(通讯员 肖鹏)近日,我校果蔬园艺作物种质创新与利用全国重点实验室、湖北洪山实验室刘继红教授团队在Plant Biotechnology Journal杂志上发表了题为“The Transcription Factors NFYA1 and GBF3 Jointly Regulate CHS2 to Promote Tangeretin Accumulation and Cold Tolerance in Citrus”的研究论文。该研究揭示了桔皮素(tangeretin)在柑橘抗寒响应中的重要作用,并探明了一个由CiNFYA1-CiGBF3-CiCHS2共同构成的关键转录调控模块,该模块通过协同激活桔皮素的生物合成显著增强柑橘耐寒性。
柑橘是世界第一大类水果,同时也是我国南方最为重要的果树作物之一,在农产品经济中占据关键地位。由于其喜温畏寒的生物学特性,低温胁迫严重制约柑橘产业的可持续发展,因此发掘抗寒遗传资源并阐明其分子机制,对抗寒新种质培育具有重要实践意义。宜昌橙(Citrus ichangenesis)作为柑橘属中具有突出耐寒性的野生资源,为挖掘抗寒相关基因及代谢物提供了理想材料。
类黄酮是一类广泛参与植物逆境应答的保护性化合物,在活性氧清除和细胞膜稳定性维持中发挥关键作用。桔皮素(4′,5,6,7,8-五甲氧基黄酮)作为类黄酮衍生物,虽在药用与营养领域价值显著,但其在植物抗逆生理中的功能机制尚未明确。查尔酮合成酶(CHS,chalcone synthase)是类黄酮生物合成途径中的限速酶,通过介导各种类黄酮产物合成参与多种非生物胁迫响应。除经典的MYB-bHLH-WD40(MBW)转录复合体外,已有研究表明WRKY、bZIP和NAC等其他转录因子也可通过MBW依赖或非依赖途径调控类黄酮合成,进而参与植物生长发育和逆境适应。刘继红教授团队前期研究发现,与低温敏感型柑橘材料HB柚相比,抗寒柑橘资源宜昌橙中桔皮素在低温胁迫下显著积累,其相关的合成基因CiCHS2受低温强烈的诱导表达,然而在低温胁迫下桔皮素的合成调控机制仍知之甚少。
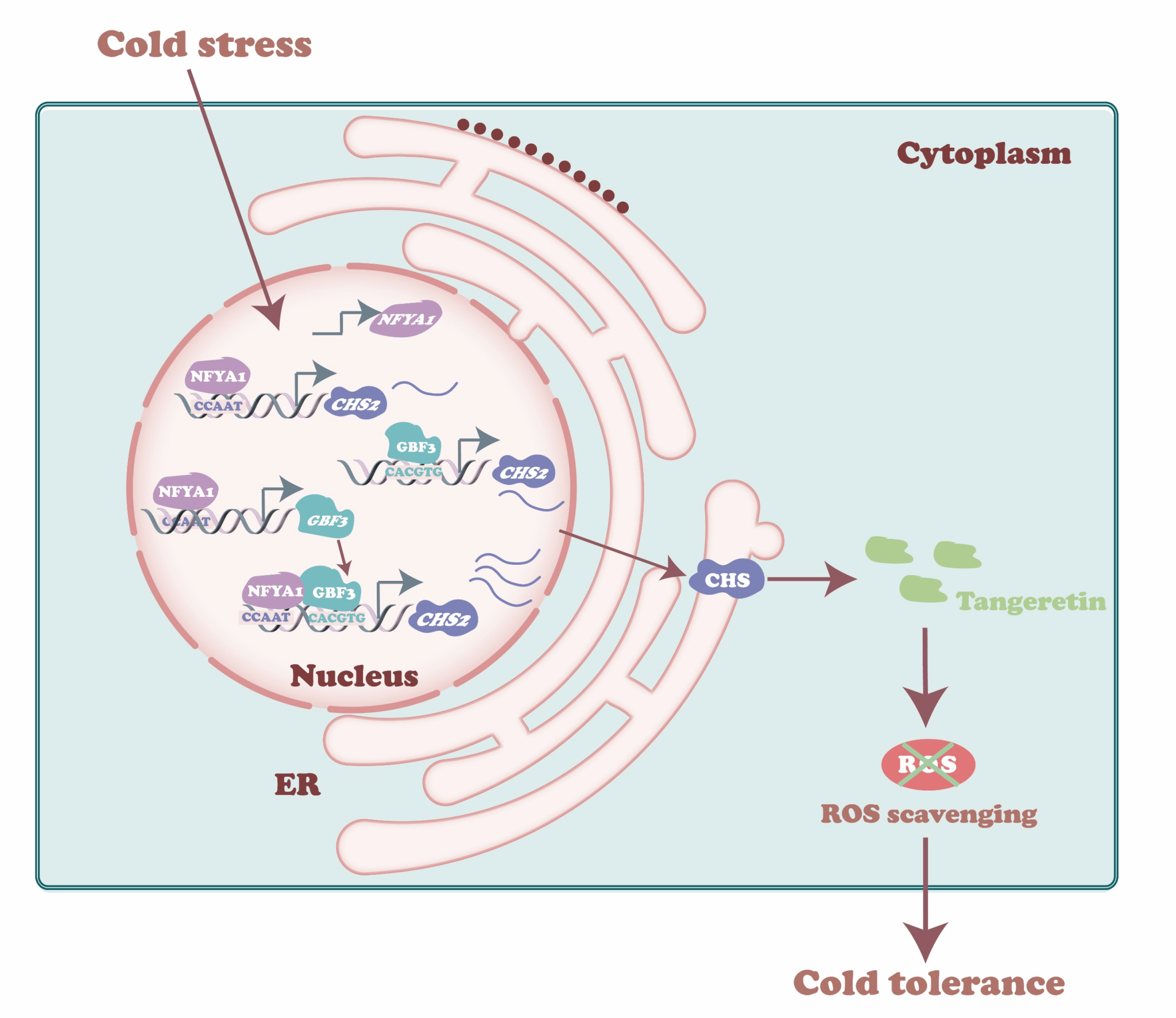
CiNFYA1-CiGBF3-CiCHS2模块调节柑橘低温应答中桔皮素积累模型
团队通过外源桔皮素处理证实了该物质可通过减轻氧化损伤有效增强柑橘耐寒性,并验证了CiCHS2通过调控桔皮素的生物合成,在耐寒性调控中发挥了积极作用。随后,为解析CiCHS2的转录调控机制,团队通过酵母单杂交筛选体系,从低温处理的宜昌橙cDNA文库中鉴定出两个转录因子:CiNFYA1(NF-Y家族)和CiGBF3(bZIP家族)。Y1H、EMSA、ChIP-qPCR和LUC等实验证明了CiNFYA1和CiGBF3可分别特异性结合CiCHS2启动子中的CCAAT和G-box作用元件来激活CiCHS2的转录表达。同时,转基因实验表明CiNFYA1和CiGBF3通过调控CiCHS2表达和桔皮素合成积累从而增强宜昌橙抗寒性。此外,研究人员还发现CiNFYA1还可直接结合CiGBF3启动子调控其表达,并与之发生蛋白互作,进一步协同强化CiCHS2的转录激活。
该研究首次揭示了一个由CiNFYA1–CiGBF3–CiCHS2组成的分级调控模块在柑橘低温胁迫响应中的重要作用,为解析类黄酮介导的植物耐寒机制提供了新视角,也为作物抗逆分子育种提供了潜在靶点与理论依据。
我校园艺林学学院刘继红教授为该论文通讯作者,已毕业的肖鹏博士为该论文第一作者,李春龙教授为本研究提供了指导和帮助。相关研究依托华中农业大学果蔬园艺作物种质创新与利用全国重点实验室平台,得到了国家重点研发计划项目、国家自然科学基金和江苏省重点研发计划项目等资助。
审核人:刘继红
论文链接:https://onlinelibrary.wiley.com/doi/10.1111/pbi.70371
英文摘要:
Tangeretin has been known as a polymethoxylated flavone conferring both phytoprotection and nutraceutical value, but cryoprotective roles and molecular regulation of tangeretin under abiotic stresses remain largely unexplored. In this study, we demonstrated that cold treatment led to greater accumulation of tangeretin and up-regulation of Chalcone Synthase 2 (CiCHS2) in Ichang papeda (Citrus ichangensis), a cold-hardy citrus species, relative to cold-sensitive genotype. CiCHS2, localized in endoplasmic reticulum, was shown to function in cold tolerance by modulating tangeretin synthesis. We revealed that the transcription factors CiNFYA1 and CiGBF3 act as transcriptional activators of CiCHS2 by interacting with CCAAT and G-box (CACGTG) element, respectively, in the gene promoter. Furthermore, CiNFYA1 could regulate CiGBF3 by binding to the gene promoter. In addition, CiNFYA1 physically interacted with CiGBF3, and the resulting protein complex further promoted transactivation of CiCHS2. Moreover, CiNFYA1 and CiGBF3 were demonstrated to play a positive role in modulation of cold tolerance by regulating CHS2-mediated tangeretin accumulation. Taken together, our findings unravel a hierarchical regulatory network wherein CiNFYA1-CiGBF3 cooperatively activated CiCHS2-dependent tangeretin biosynthesis in response to cold stress. These results advance our understanding on molecular regulation of tangeretin accumulation under cold stress and provide valuable targets for engineering cold-tolerant crops through metabolic engineering.