南湖新闻网讯(通讯员 贾明慧)近日,资源与环境学院谭文峰教授团队在Soil Biology and Biochemistry发表题为“Soil aggregates stability is evidently enhanced by super-binding of the N-terminal disordered tail of glomalin to soil minerals”的研究论文,该研究首次解析了球囊霉素同型十四聚体冷冻电镜结构,明确其N端39个氨基酸构成的无序尾巴为“超级胶结”核心,为揭示其增强土壤团聚体稳定性的分子机制提供了重要依据。
球囊霉素是由丛枝菌根真菌分泌的关键土壤蛋白,广泛存在于自然土壤中,被认为是调控土壤结构和提升土壤质量的重要生物因子。长期以来,受限于提取纯度低、结构信息不清等因素,球囊霉素在土壤结构改良中的作用机制一直未被系统阐明。本研究系统分析了球囊素的分子结构及其对土壤矿物的胶结能力,并强调其特定结构在促进土壤团聚过程中发挥重要作用(图1)。
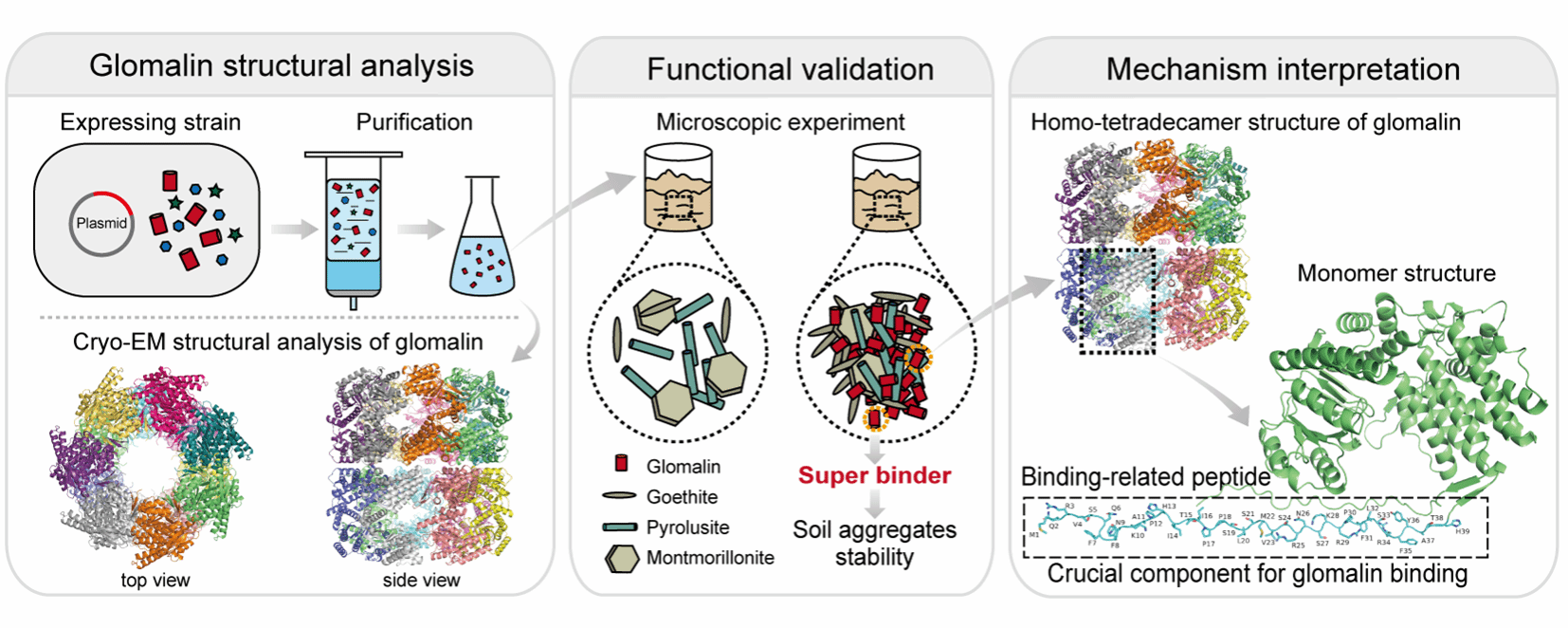
图1 球囊霉素N端胶结肽驱动超级胶结效应显著提升土壤团聚体稳定性
研究团队通过基因工程手段成功获得纯度达98%以上的球囊霉素样品,结合冷冻电镜(cryo-EM)技术解析出其同型十四聚体的笼状空间结构。微宇宙培养实验证实,球囊霉素显著增强了土壤团聚体的稳定性,其对土壤矿物的胶结力分别是腐殖酸的1-9倍、牛血清白蛋白的3-70倍。围绕其特定结构进一步研究发现N端39个氨基酸构成的无序尾巴是球囊霉素的“超级胶结”核心,它可灵活伸展并与土壤矿物表面多位点结合,从而显著提升土壤团聚体的稳定性。该研究不仅在分子层面揭示了球囊霉素改善土壤结构的关键机制,也为其作为生态型土壤改良剂的产业化开发提供了理论支撑与技术路径。
华中农业大学博士研究生贾明慧和复旦大学博士研究生王友超为论文共同第一作者,谭文峰教授为通讯作者,荷兰瓦格宁根大学Luuk Koopal教授,台湾中兴大学周三合教授,武汉理工大学方临川教授,南京林业大学冯有智教授,华中农业大学何进教授、刘玉荣教授、侯静涛副研究员和罗德华博士后等参与了该项研究工作。该研究获得了国家重点研发项目和国家自然科学基金等项目的资助。
【英文摘要】
Glomalin-related soil protein (GRSP) extracted from soil is considered crucial for the formation and stability of soil aggregates. However, due to limitations in extraction purity and interference from co-extracted products, the actual contribution of pure glomalin produced by arbuscular mycorrhizal fungi (AMF) to soil structure improvement and its specific mechanism of action remain elusive. Here, genetic engineering and cryo-electron microscopy (cryo-EM) are introduced to obtain purified glomalin and to determine its homo-tetradecamer structure. This allowed investigations of the effect of pure glomalin on soil aggregate stability and the specific glomalin-mineral interaction mechanism. The results showed that addition of glomalin significantly enhanced the formation of soil water-stable aggregates and soil macroaggregates. This enhancement was primarily attributed to the strong binding of glomalin to soil minerals, as evidenced by single molecule force spectroscopy (SMFS) and attenuated total reflectance-Fourier transform infrared spectrum (ATR-FTIR) experiments. Glomalin structural analysis, comparison of its amino sequence alignment with that of Escherichia coli heat shock protein 60 (E. coli Hsp60) and mineral binding experiments with several glomalin related mutants highlighted that the N-terminus disordered tail of glomalin composed of ~39 amino acids were crucial for the glomalin super binding ability. These findings advance the understanding of glomalin’s intrinsic mechanism for improving soil structure and open the opportunity for mass production of this ecologically important protein as a soil amendment.
论文链接:https://doi.org/10.1016/j.soilbio.2025.109908
审核人:谭文峰