南湖新闻网讯(通讯员 王文涛)近日,我校资源与环境学院土壤矿物与环境团队在南方红壤区重金属铬污染调控和修复方面取得系列进展。相关成果分别以“Chromium(VI) Adsorption and Reduction in Soils under Anoxic Conditions: The Relative Roles of Iron (oxyhr)oxides, Iron(II), Organic Matters, and Microbes”和“Iron(II/III) Alters the Relative Roles of the Microbial Byproduct and Humic Acid during Chromium(VI) Reduction and Fixation by Soil Dissolved Organic Matte”为题发表于环境领域TOP期刊Environmental Science & Technology。
土壤铬污染,已成为全球环境面临的严峻挑战。有效治理铬污染,实现受污染环境的绿色可持续修复,打赢污染防治攻坚战,需深入理解污染物在土壤中的迁移转化行为,且应结合不同区域土壤特性(铁氧化物、有机物和微生物类型和含量)进行精准修复。土壤中铬常以Cr(VI)和Cr(III)存在。Cr(VI)具有很强的溶解性和生物可利用性;而Cr(III)溶解度低,且易形成Cr(OH)₃沉淀,生物有效性低。因此,Cr(VI)还原为Cr(III)是有效的解毒途径。土壤中这一转化过程异常复杂,受微生物、有机质、活性矿物如铁氧化物和土壤本身或外源添加Fe2+介导的电子传递过程影响,但不同土壤活性组分的作用贡献尚不清楚,严重阻碍了铬污染土壤的精准治理和修复。
本研究围绕南方红壤区Cr(VI)在土壤中的转化过程,重点探讨了土壤铁氧化物、Fe(II)、有机物和微生物群落的协同作用。研究表明,土壤中铁氧化物的含量与Cr(VI)的固定量密切相关,不同类型的铁氧化物对Cr(VI)固定量存在显著差异,以水铁矿为主的土壤对Cr(VI)的固定效果明显高于富含赤铁矿和/或针铁矿土壤。在富含赤铁矿或针铁矿的土壤中,Cr(VI)的还原主要由易氧化有机碳和微生物驱动,而在富含水铁矿的稻田土壤中,Cr(VI)的还原由Fe(II)、有机质和微生物共同作用(图1)。此外,外源Fe(II)的添加显著提高了富含水铁矿和/或针铁矿土壤中Cr(VI)的还原钝化,而对富含赤铁矿土壤中Cr(VI)还原钝化的影响较小。
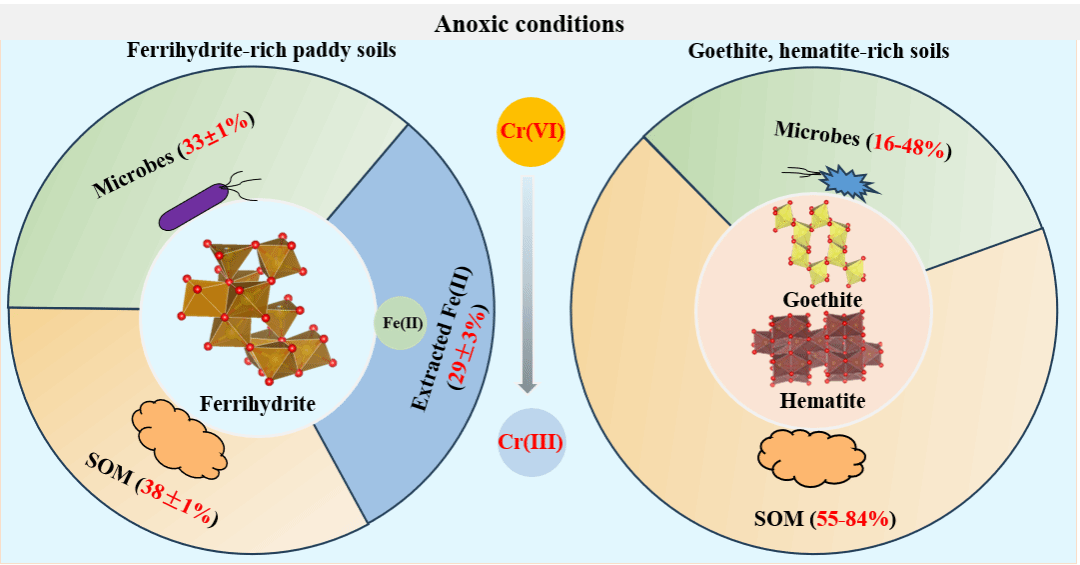
图1 淹水条件下土壤中铁氧化物、有机质及微生物对Cr(VI)还原的贡献
在此基础上,研究进一步揭示了土壤中组分不同的溶解性有机物(DOM)(如以微生物副产物为主或以腐殖酸类物质为主)在有无Fe2+/3+存在条件下还原Cr(VI)的过程和机制。以微生物副产物为主的DOM还原Cr(VI)能力显著强于以腐殖酸类物质为主DOM。然而,当存在Fe2+/3+时,DOM中腐殖酸类大分子(如单宁、木质素和富含CHON组分等)则与Fe2+/3+共沉淀形成水铁矿-有机物复合体。由于Fe2+/3+与腐殖酸类组分相互作用增加了有机物的供电子容量并促进Fe(II)生成、水铁矿-有机物复合体表面富集酚羟基、羧基、芳香族化合物和碳缺陷,以及生成羟基自由基加速腐殖酸分解为小分子和微生物副产物的矿化,显著促进了以腐殖酸类成分为主DOM中Cr(VI)的还原,而抑制了以微生物副产物为主DOM中Cr(VI)的还原。
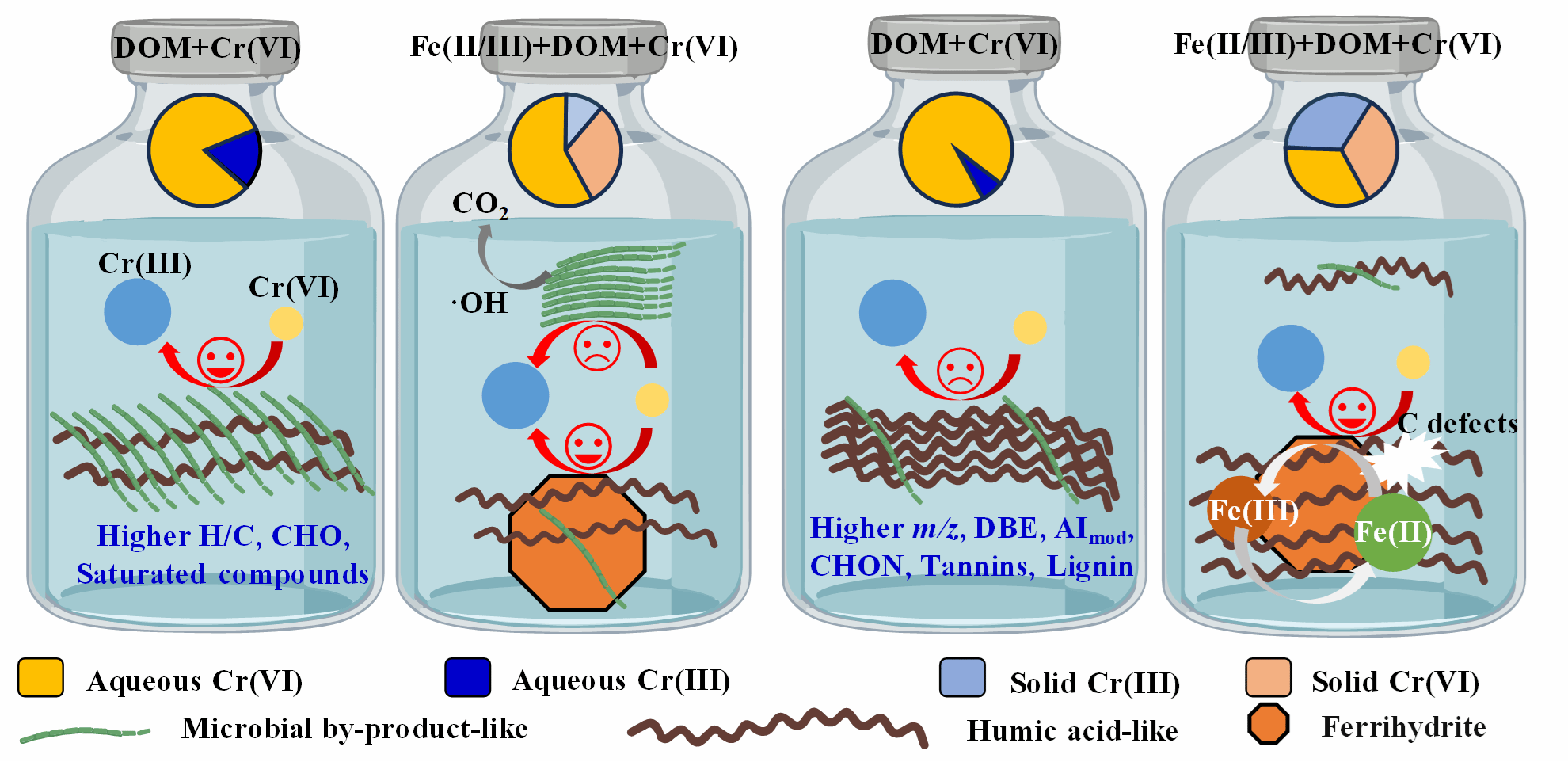
图2 富含微生物副产物和腐殖酸的DOM对Cr(VI)的还原和固定的差异
这些研究成果加深了对土壤中氧化还原活性重金属污染物的地球化学行为的理解,并为针对土壤的特定物理化学特性进行重金属污染土壤的精准高效绿色修复提供了理论指导和技术支撑。
我校资源与环境学院博士研究生王文涛为两篇论文的第一作者,殷辉副教授为共同通讯作者和唯一通讯作者,中国科学院生态环境研究中心丁龙君副研究员为第一篇论文的共同通讯作者。我校谭文峰教授和冯雄汉教授等指导了研究工作的开展。本研究得到国家自然科学基金和校自主创新基金的资助。
审核人 殷辉
论文链接: https://doi.org/10.1021/acs.est.4c08677
【英文摘要1】
Chromium (Cr) transformation in soils mediated by iron (Fe) (oxyhr)oxides, Fe(II), organic matter (OM), and microbes is largely unexplored. Here, their coupling processes and mechanisms were investigated during anoxic incubation experiments of four Cr(VI) spiked soil samples with distinct physicochemical properties from the tropical and subtropical regions of China. It demonstrates that easily oxidizable organic carbon (EOC, 55-84%) and microbes (16-48%) drive Cr(VI) reduction in soils enriched with goethite and/or hematite, among which in dryland soils microbial sulfate reduction may also be involved. In contrast, EOC (38 ± 1%), microbes (33 ± 1%), and exchangeable and poorly crystalline Fe (oxyhr)oxide-associated Fe(II) (29 ± 3%) contribute to Cr(VI) reduction in paddy soils enriched with ferrihydrite. Additionally, exogenous Fe(II) and microbes significantly enhance Cr(VI) reduction in ferrihydrite- and goethite-rich soils, and Fe(II) greatly promotes but microbes slightly inhibit Cr passivation. Both Fe(II) and microbes, especially the latter, promote OM mineralization and result in the most substantial OM loss in ferrihydrite-rich paddy soils. During the incubation, part of the ferrihydrite converts to goethite but microbes may hinder the transformation. These results provide deep insights into the geochemical fates of redox-sensitive heavy metals mediated by the complicated effects of Fe, OM, and microbes in natural and engineered environments.
论文链接2:https://doi.org/10.1021/acs.est.4c10552
【英文摘要2】
Though reduction of hexavalent chromium (Cr(VI)) to Cr(III) by dissolved organic matter (DOM) is critical for the remediation of polluted soils, the effects of DOM chemodiversity and underlying mechanisms are not fully elucidated yet. Here, Cr(VI) reduction and immobilization mediated by microbial byproduct (MBP)- and humic acid (HA)-like components in (hot) water-soluble organic matter (WSOM), (H)WSOM, from four soil samples in tropical and subtropical regions of China were investigated. It demonstrates that Cr(VI) reduction capacity decreases in the order WSOM > HWSOM and MBP-enriched DOM > HA-enriched DOM due to the higher contents of low molecular weight saturated compounds and CHO molecules in the former. The presence of Fe(II/III) selectively coprecipitates with high molecular weight components (e.g., tannins, lignin, and CHON-rich compounds) to form ferrihydrite and greatly inhibits Cr(VI) transformation and fixation in MBP-enriched DOM but enhances that in HA-enriched DOM. This is probably owing to the combined effects of (1) the increase of DOM electron-donating capacity and Fe(II) generation during the reactions of HA with Fe(II) and Fe(III), respectively; (2) the enrichment of phenolic and carboxyl groups, aromatic compounds, and carbon defects on ferrihydrite surfaces; and (3) the acceleration of HA decomposition and MBP mineralization by hydroxyl radicals. These findings enhance our understanding of the chemodiversity of soil DOM, the complex interactions between Cr(VI), DOM, and Fe(II/III), and can help design remediation strategies for contaminated environments.