南湖新闻网讯(通讯员 蔡廷卫)近日,我校植物科学技术学院农药毒理学与有害生物抗药性研究团队研究成果以“Insecticide susceptibility in a planthopper pest increases following inoculation with cultured Arsenophonus”为题在The ISME Journal发表。研究深入探讨了共生细菌Arsenophonus在水稻重要害虫褐飞虱(Nilaparvata lugens)抗药性中的作用,并为害虫抗药性治理策略提供了新的视角。
褐飞虱是水稻的重要害虫,通过刺吸汁液和传播病毒对水稻造成直接和间接危害,从而引起巨大的经济损失。研究团队前期围绕褐飞虱的抗药性问题开展系列研究,揭示了不同褐飞虱田间种群中微生物丰度及多样性与宿主杀虫剂敏感性变异的内在联系。其中,共生细菌Arsenophonus被鉴定为调控褐飞虱杀虫剂敏感性的重要成员(Zhang et al., 2023)。褐飞虱体内共生细菌Ca. Arsenophonus nilaparvatae是Arsenophonus属第三分支-猎蝽分支的成员,此前该分支共生细菌的纯培养一直是限制相关研究继续深入的难题。在褐飞虱中,研究发现共生细菌Arsenophonus的感染与褐飞虱对杀虫剂敏感性密切相关(Pang et al., 2018),这一敏感性特征为减少化学杀虫剂的使用提供了潜在途径;其次,深入理解共生细菌Arsenophonus介导的宿主杀虫剂敏感性增强机制,有望在其他害虫共生系统中推广应用,为害虫抗药性治理提供更广泛的思路;最后,通过共生体的基因工程,未来有可能开发出具备关键功能的菌株,例如使宿主对病毒传播产生抗性,从而应用于农作物病害防控。
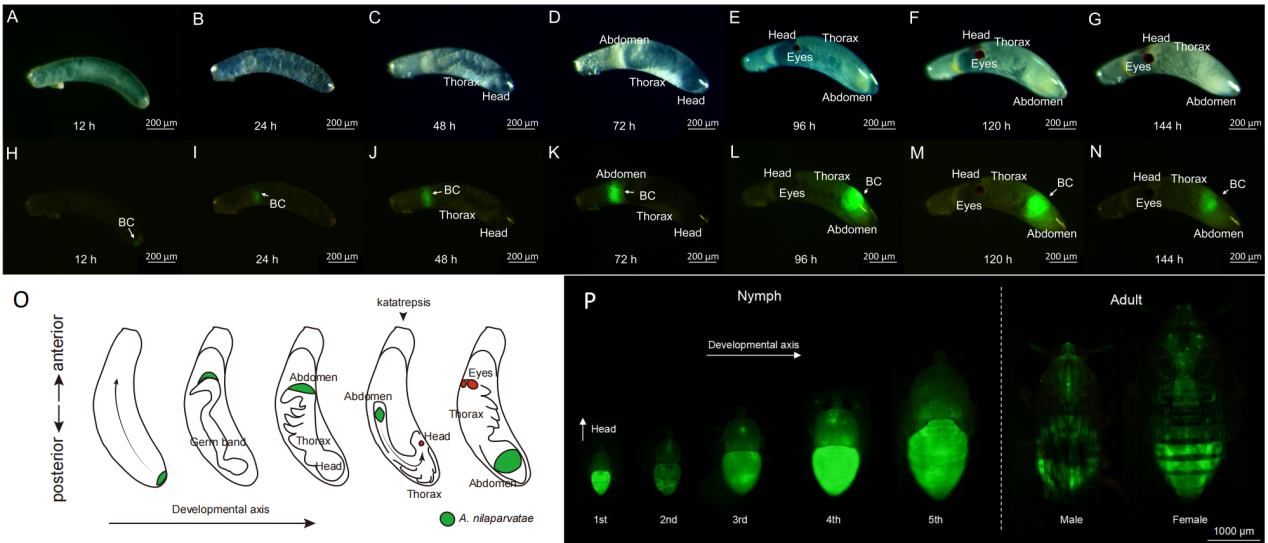
▲Ca. Arsenophonus nilaparvatae感染贯穿褐飞虱发育全程
在本研究中,研究团队首次成功建立了该微生物的纯培养方法,并在此基础上解析了其全基因组信息,此项技术的突破为进一步的功能研究奠定了重要基础。通过基因改造,使Ca. Arsenophonus nilaparvatae原位表达荧光蛋白,从而实现了该共生菌在宿主体内的感染路径追踪。通过将该改造菌株重新引入褐飞虱体内,首次详细揭示了其垂直传播与体细胞扩散模式。
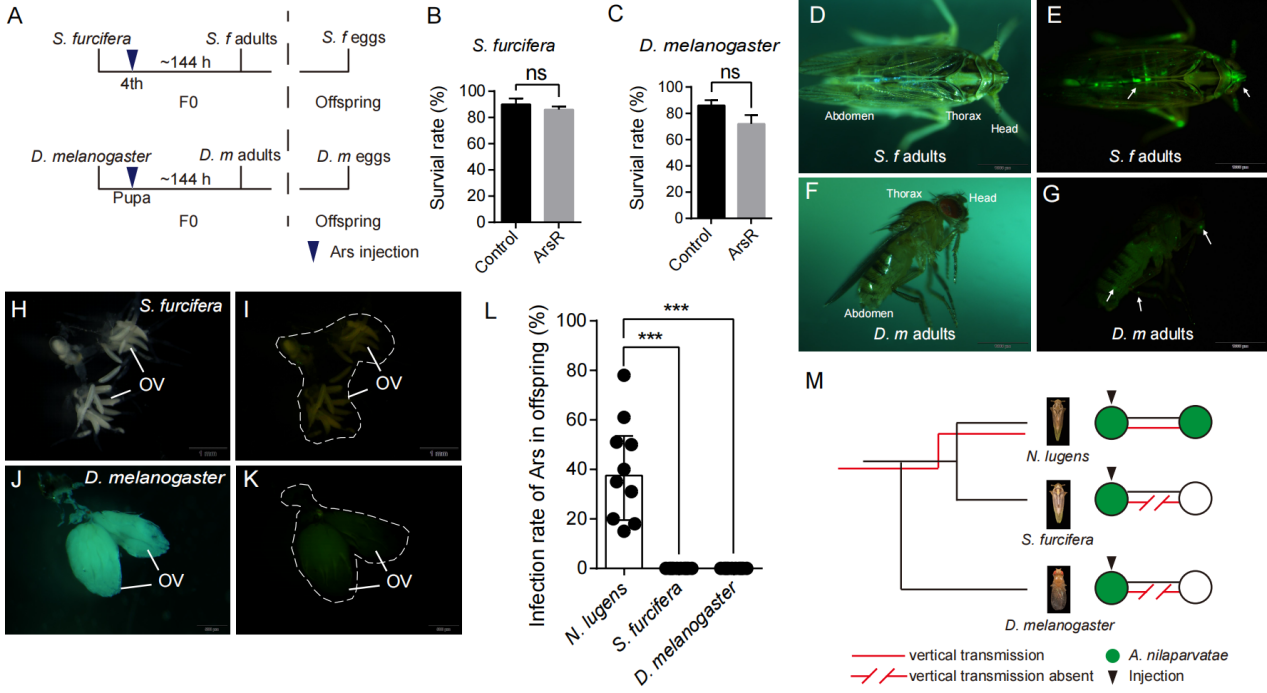
▲Ca. Arsenophonus nilaparvatae 在黑腹果蝇及白背飞虱中的适应性
同时,研究还进一步探讨了该菌株在模式生物黑腹果蝇及宿主近缘物种白背飞虱中的适应性,发现尽管其可成功定植,但无法在这些物种中维持垂直传播。更为重要的是,通过比较接种Ca. Arsenophonus nilaparvatae的褐飞虱与未处理对照组,研究清晰地验证了该共生体显著增强宿主对杀虫剂的敏感性,揭示了其在害虫抗药性治理中的潜在功能。这一发现为创新型害虫管理策略提供了新的思路,尤其是通过成功培养基因改造的Ca. Arsenophonus nilaparvatae菌株并将其重新引入害虫宿主的技术,开辟了利用共生体调控害虫对杀虫剂敏感性的新途径。为应对全球范围内日益严峻的害虫抗药性问题提供了全新思路和解决方案。
华中农业大学植物科学技术学院农药学专业博士研究生蔡廷卫为论文第一作者,我校万虎教授为通讯作者。李建洪教授、何顺副教授、利物浦大学Gregory D. D. Hurst教授以及英属哥伦比亚大学Kayla C. King教授对该研究提供了指导。以上研究得到了国家自然科学基金、英国BBSRC国际合作等项目的资助。
审核人:李建洪
英文摘要:
Facultative vertically transmitted symbionts are a common feature of insects that determine many aspects of their hosts’ phenotype. Our capacity to understand and exploit these symbioses is commonly compromised by the microbes unculturability and consequent lack of genetic tools, an impediment of particular significance for symbioses of pest and vector species. Previous work had established that insecticide susceptibility of the economically important pest of rice, the brown planthopper Nilaparvata lugens, was higher in field-collected lineages that carry Ca. Arsenophonus nilaparvatae. We established Ca. A. nilaparvatae into cell-free culture and used this to establish the complete closed genome of the symbiont. We transformed the strain to express GFP and reintroduced it to N. lugens to track infection in vivo. The symbiont established vertical transmission, generating a discrete infection focus towards the posterior pole of each N. lugens oocyte. This infection focus was retained in early embryogenesis before transition to a diffuse somatic infection in late N. lugens embryos and nymphs. We additionally generated somatic infection in novel host species, but these did not establish vertical transmission. Transinfected planthopper lines acquired the insecticide sensitivity trait, with associated downregulation of the P450 xenobiotic detoxification system of the host. Our results causally establish the role of the symbiont in increasing host insecticide sensitivity with implications for insecticide use and stewardship. Further, the culturability and transformation of this intracellular symbiont, combined with its ease of reintroduction to planthopper hosts, enables novel approaches both for research into symbiosis and into control of insect pest species.
论文链接:https://doi.org/10.1093/ismejo/wrae194